Metals of different potentials come into contact with each other when an electrolyte is present, and a chemical reaction occurs when a current flows from a high-potential metal to a low-potential metal. The greater the potential difference between different metals, the stronger the current, and the faster and more severe the corrosion. The chemical reaction must take place with the electrolyte (ie the medium through which the current passes).
The most common electrolytes are caused by weather or climatic conditions such as humidity, fog, dew, rain, condensation. Temperature also affects the rate of electrochemical reactions. In general, the higher the temperature, the faster the chemical reaction. When different metals encounter electrolytes and contact and produce galvanic corrosion, one of the metals will corrode faster than normal, while the other metal will slow down or will not corrode at all. The table below lists the potentials of different base metals and their alloy materials.
When the two metals are put together, the cathode (ie, the one with the higher potential) will not be affected by the corrosion, and the lower potential, that is, the anode will corrode. And alloy potential sequence table anode corrosion end (anode or lowest potential end) magnesium, magnesium alloy, zinc aluminum no, fu, aluminum, steel or iron, cast iron ferrochrome (active), nickel-resistant cast iron 3 type stainless steel (active), 316 stainless steel (active) lead solder, lead, tin, nickel (active), Inconel (active), Hastel C nickel-based corrosion-resistant alloy (active), stress corrosion stress corrosion is the least understood, It is also one of the most dangerous causes of fastener breakage.
Common causes include stress corrosion cracking, hydrogen embrittlement, hydrogen accelerated stress corrosion cracking, and stress embrittlement. The identity between these fracture mechanisms is much more important than the difference. However, only corrosion stress cracking and hydrogen accelerated stress corrosion cracking can be caused. Fast-stressed fasteners are prone to stress corrosion cracking or hydrogen accelerated stress corrosion cracking, causing cracking of the fastener. Fasteners that are subjected to tensile loads may experience stress corrosion cracking immediately.
It is also possible that stress corrosion cracking does not occur after weeks, months or even years. Stress corrosion cracking often occurs without warning, and once it occurs, the consequences can be severe. When the tensioned fastener encounters conditions that induce corrosion, stress corrosion cracking may occur. The specific process of stress corrosion cracking is not fully understood, but experts have found that when corrosion occurs, fine cracks appear in the high stress areas. This crack is caused by residual stress in the production process or external load.
When the surface encounters high stress, the local corrosion process is aggravated. Under the dual action of stress and corrosion, the crack expands inward, thereby reducing the cross-sectional area. Finally, when the remaining cross-sectional area cannot support the load, the fastener will break. The rate of fracture depends on the magnitude of the stress, the degree of corrosion and the metallurgical properties of the fastener. Hydrogen accelerated stress corrosion cracking is similar to stress corrosion cracking. In use, the hydrogen produced by the corrosion process accelerates the stress corrosion cracking. This phenomenon is called hydrogen accelerated stress corrosion cracking.
For example, zinc and iron in galvanic couples produce hydrogen when they encounter water. If corrosion has occurred and small cracks are present, hydrogen will accelerate the crack. Even fasteners with stress corrosion resistance, such as hydrogen generated during use, penetrate below the surface of the fastener, causing fracture. Some fracture analysis reports that this phenomenon of fracture due to hydrogen generated by the corrosion process is called "hydrogen embrittlement". Hydrogen embrittlement is caused by the inhalation of hydrogen into the fastener material during the manufacturing process. Hydrogen is generated during acid etching and alkali cleaning prior to electroplating and subsequent electroplating, and hydrogen accumulates in the metallized fasteners.
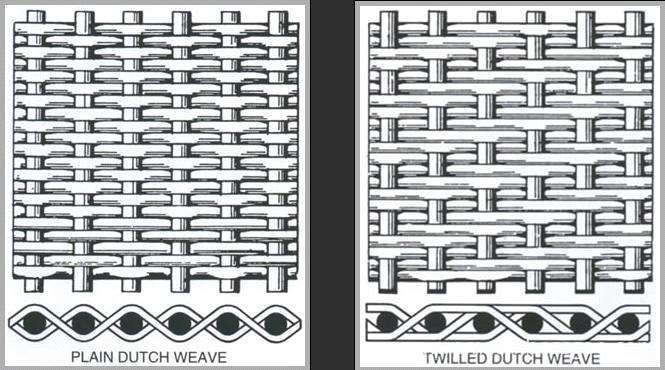
Stainless Steel Plain Dutch Weave Mesh, also known as wire mesh, it is made of high quality stainless steel wire. Similar to plain weave, it is weaved line by line. But for this kind of Stainless Steel Wire Cloth, the difference is the diameter of the weft is more thicker than the diameter of the warp. The weft wire is close together and then form a conical or wedge shaped hole. There is a big contrast between warp and weft wire diameter and density, so the net thickness and filtration precision and service life is significantly improved than common steel mesh. the filtering precision is running cannot be achieved for plain weave and Twill Weave Mesh screen.
Weaving: There are two kinds of weaving technology of Dutch woven Stainless Steel Mesh . One is plain Dutch knitting and the other is twill Dutch knitting. The weaving technology of Dutch woven stainless steel mesh combines Dutch knitting and twill weaving techniques. The coarser thread interleaved the finer threads, weaving each thread through the weft. The weft wire is closely aligned to form a cone or wedge open hole.
Features: Dutch woven stainless steel mesh features 4 points:
1."Zero" mesh, the warp wire diameter than the weft to the larger diameter.
2. Each weft is very tight
3. High precision, high liquidity
4. Filter more solid particles than square or rectangular mesh holes
Material: 201,302,304 (L), 310,316 (L), etc
Application: The Dutch weave Stainless Steel Wire Mesh is one of the best filter medium in hydraulic system, especially the spatial filtering critical applications, as well as the fuel and combustion chamber filter, sediment filter, vacuum filter, etc.
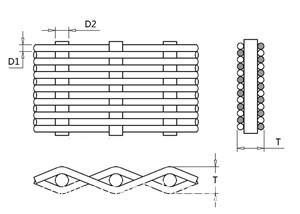
The warp wire (D1) : all longitudinal braided lines.
The weft wire (D2) : all horizontal weaving lines.
Aperture : the distance between two meridians or two weft.
Mesh number:number of mesh holes per inch.
Thickness (T) : thickness of wire mesh.
Specifiaction list:
Plain
Dutch Woven Stainless Steel Mesh
SPEC
NUMBER OF
HOLES PER INCH
WIRE DIAMETER
APERTURE(micron)
40
10mesh x 64mesh
0.55x0.42
260
50
12mesh x 75mesh
0.45x0.35
220
80
24mesh x 110mesh
0.35x0.25
160
100
25mesh x 140mesh
0.28x0.20
100
120
30mesh x 150mesh
0.25x0.18
80
140
35mesh x 180mesh
0.20x0.16
70
160
40mesh x 200mesh
0.18x0.13
60
180
45mesh x 220mesh
0.16x0.12
56
200
50mesh x 250mesh
0.15x0.11
50
240
60mesh x 300mesh
0.14x0.09
45
260
65mesh x 320mesh
0.13x0.08
36
280
70mesh x 400mesh
0.125x0.07
34
300
80mesh x 700mesh
0.11x0.039
32
Stainless Steel Plain Dutch Weave Mesh
Stainless Steel Plain Dutch Weave Mesh,Plain Dutch Weave Mesh,Fine Metal Mesh,Stainless Steel Woven Wire Mesh
Anping Xinzheng Metal Wire Mesh Co., Ltd , http://www.sievingmesh.com
