In May of this year, an article titled "Water-hydrogen engines off the assembly line in Nanyang, the Secretary of the Municipal Party Committee praised! The article on the website sparked heated discussion on the Internet. The report stated: "The Henan Nanyang Hydrogen Energy Automobile Project has achieved the latest results, and vehicles only need to add water to drive." This very "future sci-fi sense" announcement instantly caused a hot discussion among netizens, or, in a moment, netizens questioned. The questioners also included a large number of industry experts, and the focus of everyone's question was "vehicle Is the technology of "converting water into hydrogen energy in real time" really as Pang Qingnian said, has it reached a practical level?
Of course, this issue is not the focus of the discussion in this article. The protagonist of this article is a group of scientists from Trinity College in Dublin, Ireland. The research they are currently working on may be able to make the mysterious concept of the "water hydrogen engine" a reality.

â–² From left to right, Professor Max Garcia-Merkel and doctoral student Michael Craig, their team is exploring the secret of the "hydrogen hydration" catalyst (Source: Trinity College Dublin)
The research team from Trinity College Dublin is working to find a major problem facing the current production of clean energy "hydrogen energy": catalysts. They have invested a lot of time and energy in the research topic of "how to efficiently hydrate water into hydrogen through appropriate catalysts", and have recently made breakthrough progress.
The researchers published a new paper in "Nature Communications", which stated that "the main obstacle to achieving large-scale hydrogen production by electrochemical water decomposition is that the cost of the existing oxygen evolution reaction catalyst is too high and the efficiency is too low," And their research team conducted research on this topic and made new discoveries.
Water is arguably the most abundant compound in the cosmic space we have observed. One of the important reasons is that it is composed of the two most common and abundant elements in the world. The other main reason is that it is The structure is extremely stable and difficult to destroy. And the earth can also be said to be the most suitable place for stable liquid water-our temperature and atmospheric pressure combination is just right for the stable existence of liquid water-exploration in recent years has shown that water also exists on Mars, and scientists often express this Confusion, one of the important reasons is that the space environment on Mars is very different from the Earth (arguably the fast-forward version of the Earth), so it is difficult for them to imagine why there is liquid life-sustaining water on Mars.
No beakers, only computers-how do researchers do experiments?
Although there are many elements / substances that can effectively decompose relatively stable liquid water, such as ruthenium or iridium (you can find these two precious metals from the periodic table), for laboratories, the prices of these precious metals are also true It is "expensive". If you only do one or two experiments, the researchers may still be able to afford it, but if you use this rare element in the production of large-scale hydrogen hydrate, its cost is absolutely far Exceeds the standard of actual commercialization. In fact, no one in the water-to-hydrogen industry has yet discovered those cost-effective, highly available, and reliable catalysts.
So, what methods do researchers use to conduct research? Lab coat? Black-rimmed glasses? Large-caliber beaker or strange smell of "rotten eggs"? I must interrupt you before you imagine some kind of difficult laboratory research environment, because the researchers at Trinity College just sit at the desk like you and me, and hit the mouse and keyboard to complete all the "experimental work" .
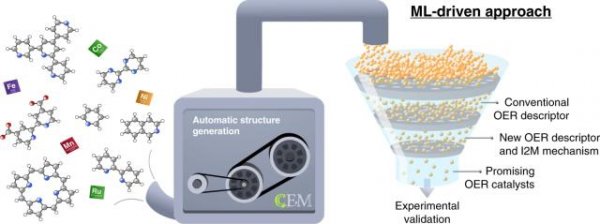
Yes, chemists and theoretical physicists from Trinity College came together to combine novel intelligent chemistry and powerful computer capabilities, and to find potential solutions that could become the "holy grail" of hydrogen-to-water catalysts Program.
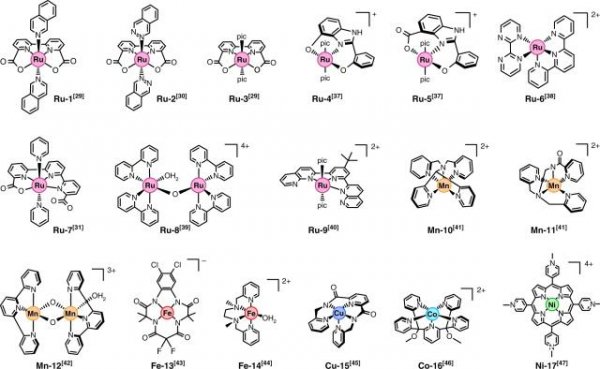
â–² Researchers study the hydrogen evolution reaction in different processes
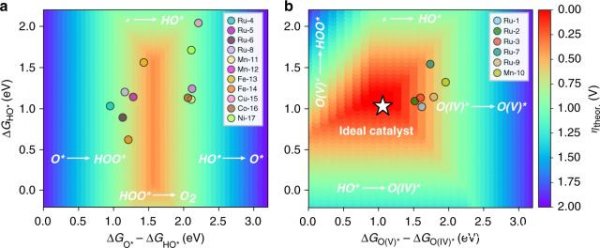
This research group led by Professor Marx Garcia-Merkel mentioned above has made a breakthrough discovery in the study of hydrogen production from water through high-performance computer experiments: the existing research has always underestimated some more With the efficiency of active catalysts, the barrier to "hydrogen overpotential" seems to have an easier way to overcome. In addition, while improving a widely accepted theoretical model for predicting the efficiency of water-to-hydrogen catalysts, they also found that supercomputers were extremely easy to find the previously elusive "green bullet" catalyst process.
â–² The research team proposed a feasible solution to accelerate the discovery of hydrogen evolution reaction (OER) catalyst
The simulation process of supercomputers can greatly speed up the process of most existing chemical experiments. The use of powerful computing models also provides scientists with a "new perspective". With supercomputers, they can quickly identify what is effective, What is invalid, although many results need to be further confirmed by actual experiments, but there is no doubt that the computer can indicate the way forward for them.
Why is hydrogen energy so important? Or, why is the research on this new clean energy so compelling? In fact, hydrogen is a potential solution for green energy. It can provide power for vehicles such as automobiles and many other application fields. Compared with other renewable fuels, hydrogen energy may be the most promising to obtain on a large scale and is the "most One of the "clean" fuels. About 10 years ago, on the "Top Gear" show, James May tested a hydrogen-powered Honda car. The only by-product produced after the combustion of its engine was water.
Perhaps in the near future, the water-hydrogen engine can really become a reality. Before that, we should calm down and invest more energy in basic research, rather than promoting certain concepts of "perpetual motion." (Text / produced with clamp)
The efficient and economical Fiber Laser Cutting Machine adopts international advanced fiber lasers, speed reducers, servo motors, gears, racks and the like, has high precision and stable performance, can cut various metal plates, and is mainly suitable for fast cutting of stainless steel, carbon steel, manganese steel, copper plates, galvanized plates, various alloy plates, rare metals and other materials. Applicable industries: sheet metal, precision instruments, mechanical equipment, metal molds, kitchen and toilet products, auto parts, craft gifts, jewelry, glasses, lighting, mobile phone communication, digital products, electronic components, clocks, computer parts, instruments, etc.
Technical Parameters
|
Type |
CE3015 |
CE4015 |
CE6015 |
CE4020 |
CE6020 |
CE4025 |
CE6025 |
|
Effective cutting width (mm) |
1500 |
1500 |
1500 |
2000 |
2000 |
2500 |
2500 |
|
Effective cutting Length (mm) |
3000 |
4000 |
6000 |
4000 |
6000 |
4000 |
6000 |
|
Range of vertical stroke (mm) |
0-80 |
||||||
|
Input power |
AC380V/50Hz;AC220V/50Hz |
||||||
|
Cutting thickness (mm) |
Accroding to Laser power |
||||||
|
Cutting speed(mm/min) |
21000 (1000W/stainless δ1mm) |
||||||
|
Idle speed(mm/min) |
100000 |
||||||
|
Maximum Acceleration (G) |
1.2 |
||||||
|
Position repeat accuracy (mm) |
±0.05 |
||||||
|
Laser power(W) |
(≤4000W)Accroding to the requriments |
||||||
|
Drive mode |
Precision rack bilateral drive |
||||||
|
Laser wavelength (nm) |
1080 |
||||||
|
Cooling mode |
water-cooling |
||||||
|
Environmental temperature |
5-35℃ |
||||||
|
Cutting material |
Carbon steel, stainless steel, alloy steel, copper, aluminum, galvanized sheet |
||||||
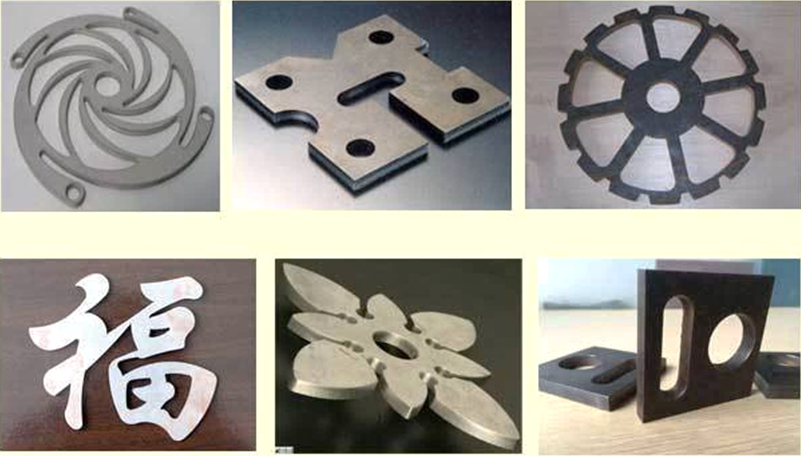
Carbon Steel Plate Cutting Machine
Steel Plate Cutting Machine,Carbon Steel Laser Cutting Machine,Carbon Steel Gantry Cutting Machine,Carbon Steel Plate Cutting Machine
Shandong Buluoer Intelligent Technology Co., Ltd. , https://www.buluoercuttingmachine.com
